Natural Selection in Drosophila

AUSTRALIAN CURRICULUM ALIGNMENT
-
Natural selection occurs when selection pressures in the environment confer a selective advantage on a specific phenotype to enhance its survival and reproduction; this results in changes in allele frequency in specific phenotype to enhance its survival and the gene pool of a population (ACSBL090)
BACKGROUND
Natural selection results from differential survival and reproduction of individuals due to differences in phenotype. A key mechanism of evolution, this change in the heritable traits occurs in a population over generations. Within any population, genetic variation will exist due to mutations. Mutations are changes in the DNA sequence of an organism and can provide organisms with either an advantage or disadvantage. Mutations that disadvantage an individual may reduce their fitness, and therefore their ability to survive and reproduce. Lowered chances of organism survival and reproduction, means their genetic material, along with the mutations are less likely to be passed on to the next generation. Traits of this nature can be referred to as “selected against”. However, there is an abundance of mutations of advantageous “selected for” traits that provide organisms with survival and reproductive advantage. These traits have a greater chance of being passed on through reproduction and therefore drive evolution. Survival of a trait is not solely dependent on sexual selection, it is also heavily influenced by predators and pathogens or environmental conditions of temperature, radiation, chemistry, or moisture.
In this investigation, students observe natural selection in action by performing a cross of White and Red-eyed Drosophila (fruit flies). Drosophila are the perfect organisms to study genetics due to their accessibility, short twelve day generation time and four chromosomes. Students begin their genetic study with male and females of two different eye colours and predict expected filial phenotype ratios with the given parental genotypes. Once students have made their predictions, they are tasked with setting up a genetic cross between red and White-eyed flies. Students then sort the offspring by eye colour and compare their ratio predictions with actual results; using the collected data as evidence to determine whether natural selection influenced the population. The increase in the number of Red-eyed flies observable over the generations serves as evidence of natural selection.
PREPARATION - BY LAB TECHNICIAN
- Add 70% ethanol or detergent to tap water in sealable containers.
-
One week before students begin the cross, you will need to ‘clear’ your Drosophila vials of all adults.
- To do this, anaesthetise all adults as per the instructions of the FlyNap® kit and dispose of them in the morgue.
- The remaining pupae will be the students' parental generation.
- To prepare thirty-two vials, add a measure of your medium powder to each vial. Then add an equal amount of tap water.
- Label the prepared vials with eight “Experimental Males,” eight “Control Males,” vials and sixteen vials of “White-eyed Females.”
- Add four to six yeast grains onto the surface of the medium in each vial. Excess amounts of yeast may damage the cultures, so be careful not to add too much. After one minute (or more) of mixing, introduce flies to the medium.
- After the Drosophila have been introduced to the medium for a few hours, clear and anaesthetise the White-eyed and Red-eyed flies.
- Using a stereomicroscope and paint brush, sort the flies by sex and type. Ensure the flies from each vial are kept separate.
- Place five anaesthetised White-eyed males and one Red-eyed Male into each of the eight prepared “Experimental Males” vials. Ensure no female flies are included with the males, as this will create unreliable results, if unsure, do not include the fly in the cross. Maintain vials on their sides until the flies awaken from anaesthesia; otherwise, the flies may become stuck in the medium.
- Place five White-eyed males into each of the eight “Control Males” vials and place five virgin White-eyed Females into each of the sixteen “White-eyed Females” vials.
Preparing Workstations
- Provide each student workstation with:
- 1 Vial with five White-eyed males (Control Males)
- 1 Vial with five white- and one Red-eyed Male (Experimental Males)
- 2 Vials with five White-eyed Virgin Females
- 2 Vials of Fresh Medium
- Prepared Morgue
- FlyNap® Anesthetizer
- White Paper
- Paint Brush
METHOD - STUDENT ACTIVITY
Setting up Genetic Cross (Week One)
-
Collect two fresh vials of medium and label one, “EC” for Experimental Cross, and the other, “CC” for Control Cross.
- Collect the required flies from your teacher. A week prior, your teacher removed the adult flies to ensure that the female flies have never mated.
- Transfer one vial of five White-eyed virgin females and one vial of White-eyed males into the Control Cross vial.
- To transfer flies, hold the two vials containing the Drosophila upright and simultaneously tap their bottoms against a hard surface lightly. Then quickly remove the plugs from the vials, and tap once again to ensure the flies remain in the bottom. Invert one vial until the mouth of the vial is flush with the mouth of the vial beneath it. Gently tap the bottom of the lower vial twice more and then quickly plug the lower vial, which now houses all the flies.
- Using the same technique, transfer one vial of White-eyed virgin females, one vial of five White-eyed males and one Red-eyed Male into the Experimental Cross vial.
- Fill in Table 1 with your predictions of the phenotypic ratio you expect to observe in each cross.
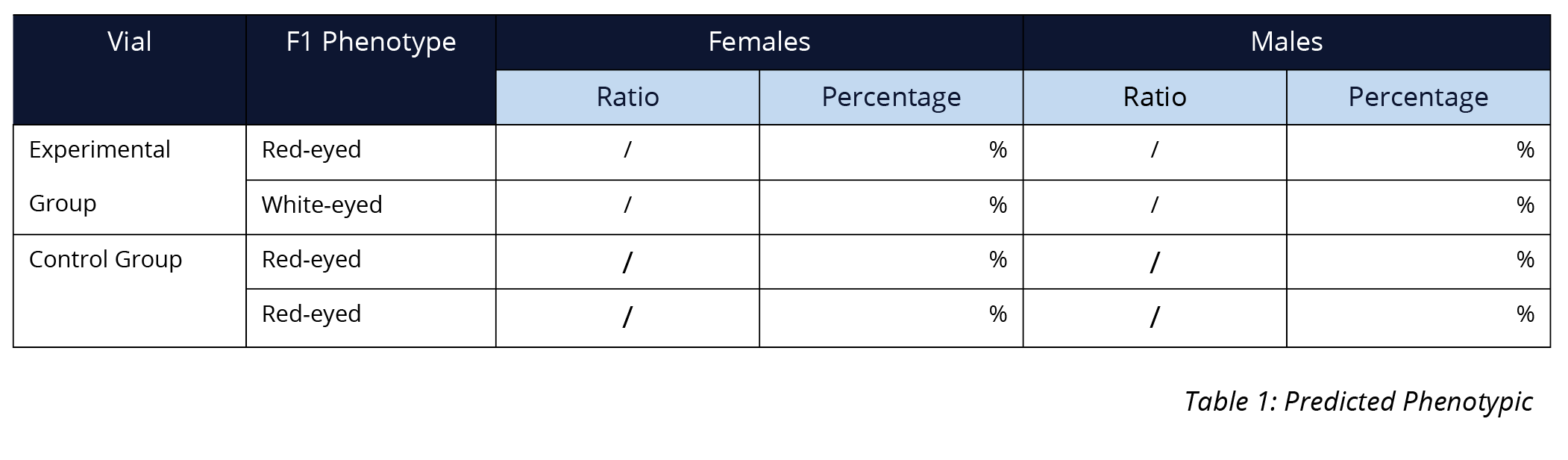
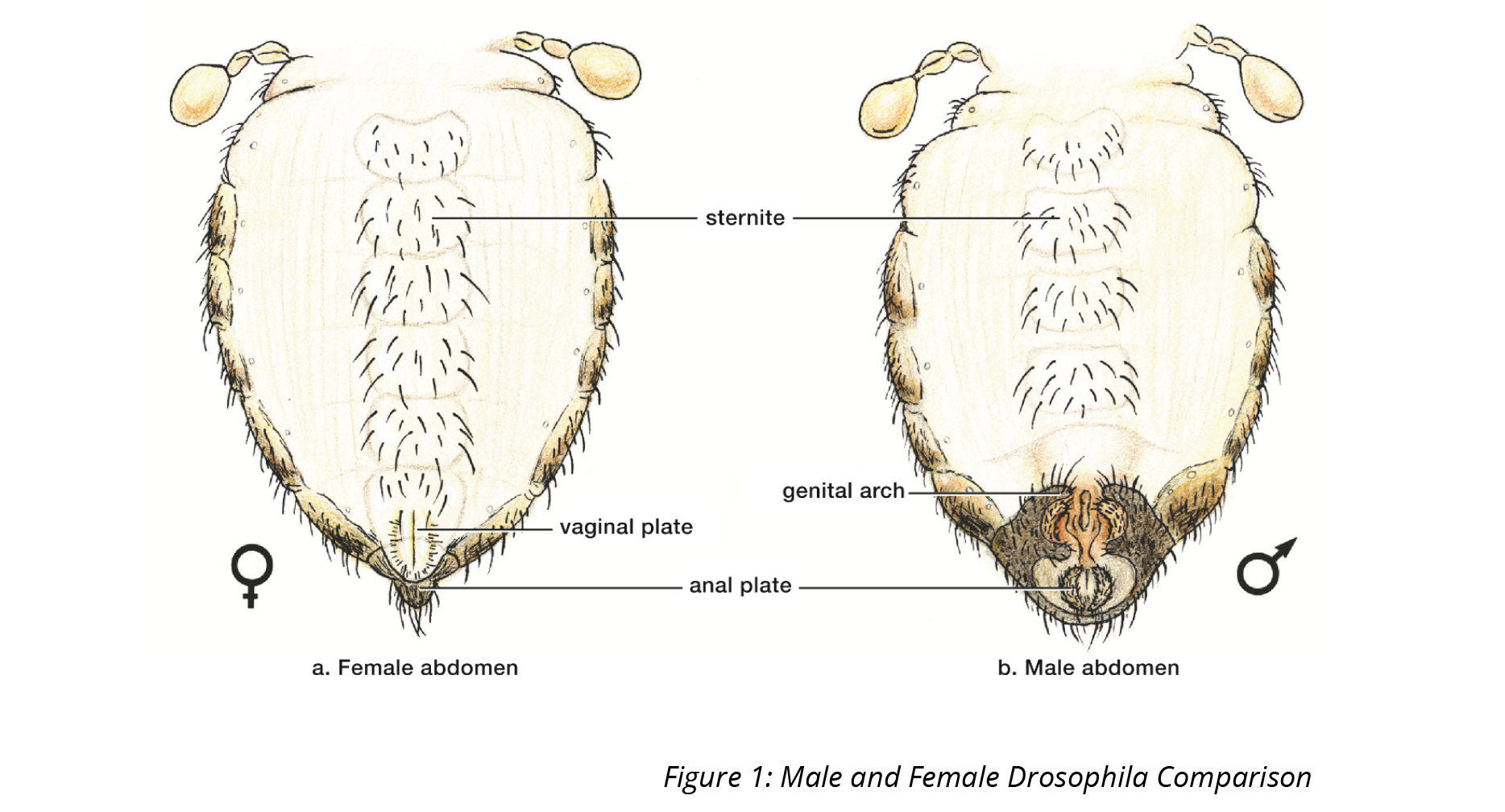
- Look for signs of mating behaviour. You may see males chasing females during courting. Observe if there is any mating behaviour that is associated with eye colour. To identify males and females, the shape and colour of the posterior portion of the abdomen can be used as a guide (Figure 1). On mature males you will see a darker, more rounded posterior compared to the more angular posterior of females. The sex combs located on their forelegs of males can also be used to identify them. However, they can be difficult to find, making them a somewhat unreliable tell among novices. You may find male flies are typically smaller than females, however this is not always the case.
- Record any mating behaviour and any behavioural differences you observed between Red-eyed and White-eyed flies.
Clearing vials of P Generation (Week Two)
-
One week after setting up Genetic cross you are required to clear the vials of the P Generation. Using FlyNap, anaesthetise the P generation flies, according to the product instructions.
- Once anaesthetised, remove the P generation adults from the experimental cross. This “clearing” of the vials is done to prevent generational crossover; parents mating with their own offspring.
- Place the anaesthetised flies into a prepared morgue.
- You should now be able to observe the F1 generation in the vial as eggs and larvae. Pay attention to the trails left by the larvae in the medium. It may also be possible for you to distinguish the larvae’s black mouthparts as they move through the medium.
- Observe the behaviour of the larvae. Take note of any morphological differences among the flies at the larval stage.
Sorting and Counting Drosophila (Week Three)
- Two weeks after setting up Genetic cross draw a grid to sort the Drosophila on white paper. Divide the paper into quadrants: White-eyed male, White-eyed female, Red-eyed Males and Red-eyed female.
-
Using FlyNap, anaesthetise F1 generation flies, according to the product instructions.
- Using a paint brush, sort the flies according to the grid and fill in the table below with the data you collected and fill in Table 2 with your results. Compare your findings with the class.
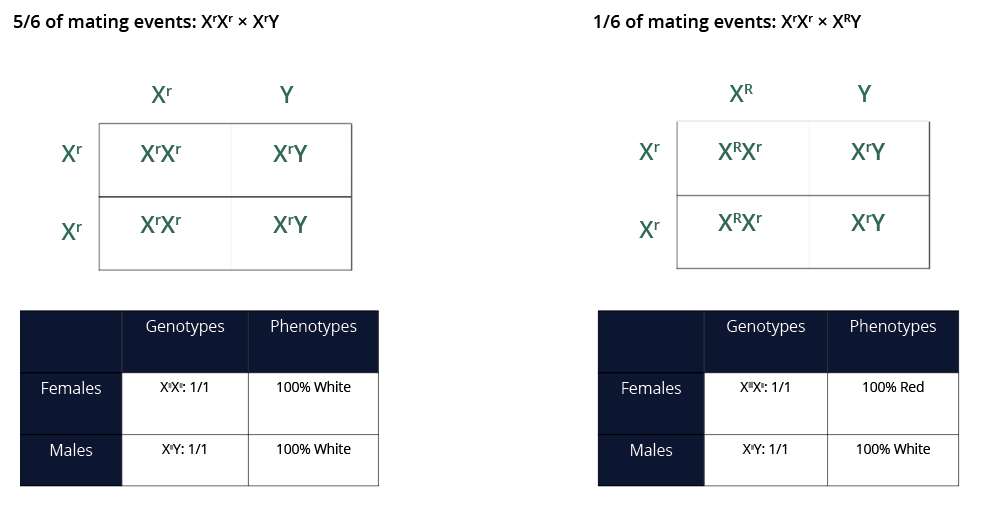
- Using two Punnett squares, explain how the results of the parental cross were achieved. What are the expected genotypic and phenotypic ratios of the F1 generation for the experimental vial, according to these Punnett squares? Remember, you began the cross with five White-eyed males, one Red-eyed Male, and five White-eyed Females.
OBSERVATIONS AND RESULTS
Student predictions will vary. Some students may opt to complete Punnett square to accurately predict the phenotypic ratio. Students should observe some mating behaviour among the population. Students may see males chasing females during courting. They may also see the male extending one wing and vibrating it from behind the female to attract it. Students may also observe copulation. Students may note that the Red-eyed Males are more active than White-eyed males. They may also see they are more likely to mate. This is likely the result of the Red-eyed flies’ greater vision. This illustrates a beneficial mutation in the red-eye gene variant and shows greater fitness.
Clearing vials of P Generation
Larvae frequently feed and perform undulating movements. Regarding eye colour, students will not be able to observe any morphological differences. Additionally, the sex of the larvae is not evident during this stage.
Sorting and Counting Drosophila
If White-eyed and Red-eyed Males mate with equal success, White-eyed males will mate with White-eyed Females for 5/6 or 83% of the total mating events. Because there is one Red-eyed Male with five White-eyed males, the Red-eyed Male should mate with a White-eyed female for only 1/6 or 17% of the total mating events.
INVESTIGATIONS
- Ask student whether they believe that the Red-eyed Drosophila have a mating advantage or disadvantage over White-eyed Drosophila based on their observations of the flies courtship and mating. Students' observations of courtship or copulation will vary. However, students may observe Red-eyed Males are typically more active and more likely to mate, due to their greater vision and thus determine the red-eye gene variant is a “beneficial mutation”. Students may also observe a higher ratio of Red-eyed to White-eyed flies than they predicted in their Punnett squares, indicating a fitness advantage in the Red-eyed phenotype.
- Ask students why they think traits which are "selected against" continue to occur in populations. The alleles for a trait do not disappear from a population just because it is "selected against". The trait can exist but not be expressed in individuals who are carriers of the trait. In the cross students conducted, two heterozygous carriers have a 25% chance of producing offspring who express the double recessive trait, and a 50% chance of having heterozygous carriers offspring.
-
Consider colour-blindness in humans. It occurs in 1 in every 12 males, yet only 1 in 200 females expresses the trait. Challenge students to describe what type of trait is colour blindness is. If colour blindness is carried on a recessive allele, b, what are the genotypes of colour-blind males and females?
- Regarding natural selection, explore how eye colour in Drosophila differs from humans.
EXTENSION EXERCISES
- In this investigation, students observed an example of a sex-linked cross. Ask students to research examples of other sex-linked traits among different species. If studying humans, colour blindness and haemophilia are two great examples of sex-linked traits in humans. Ask students to present the information to the class.
- You may like to task students with conducting additional crosses with the same batch of flies to produce F2, F3, and F4 generations. This would allow students to gain a deeper understanding of the mode of inheritance over more generations. Ask students to predict the outcomes of the crosses and compare them with observed results.
TEACHER TIP
Ask students to go through the motions of the procedure before actually performing transfers. This will ensure students feel confident conducting the procedure and will prevent nervous or tentative students from panicking or freezing when flies try to escape.
 Time Requirements
Time Requirements
- 50 mins
 Material List
Material List
- 1 Vial with five white- and one Red-eyed Male (Experimental Males)
- 2 Vials with five White-eyed Virgin Females
- 2 Vials of Fresh Medium
- Prepared Morgue
- FlyNap® Anesthetizer
- White Paper
- Paint Brush
 Safety Requirements
Safety Requirements
-
Wear appropriate Personal Protective Equipment (PPE), including gloves and lab coat.
- Wash hands before and after handling flies.
- When using FlyNap® anesthetizer, wear disposable lab gloves and work in an open area with adequate ventilation.
