Observing Slime Mould
AUSTRALIAN CURRICULUM ALIGNMENT
-
Movement of materials across membranes occurs via diffusion, osmosis, active transport and/or endocytosis
BACKGROUND
Physarum Polycephalum is a slime mould that can be found in a variety of cool, humid and dark environments; such as leaf litter. Classification of Physarum has been difficult as it possesses characteristics found across taxonomic categories; however, it can be said to belong to the Amoebozoa, the Mycetozoa, or the Myxomycetes. Physarum is a great model in the studies of cell differentiation, cytoplasmic streaming, cell cycle regulation, cytoskeletal rearrangement, mitosis, and meiosis. Many of the life processes of this mould mirror those of more complex organisms. The form of Physarum changes over its life-cycle as it adapts to different environments. The ability of Physarum to navigate among many complex strategies to find food, to form networks between different sources of food, and to modify those networks to maintain efficiencies, has drawn great interest among those who study networks and artificial intelligence. During its life cycle, Physarum takes different forms including plasmodial form. A Physarum plasmodium exists as a single large cell containing multiple diploid nuclei that replicate their DNA and divide synchronously. A single plasmodium may contain as many as 1010 nuclei. Under lab conditions, plasmodium has grown to more than 30cm in diameter.
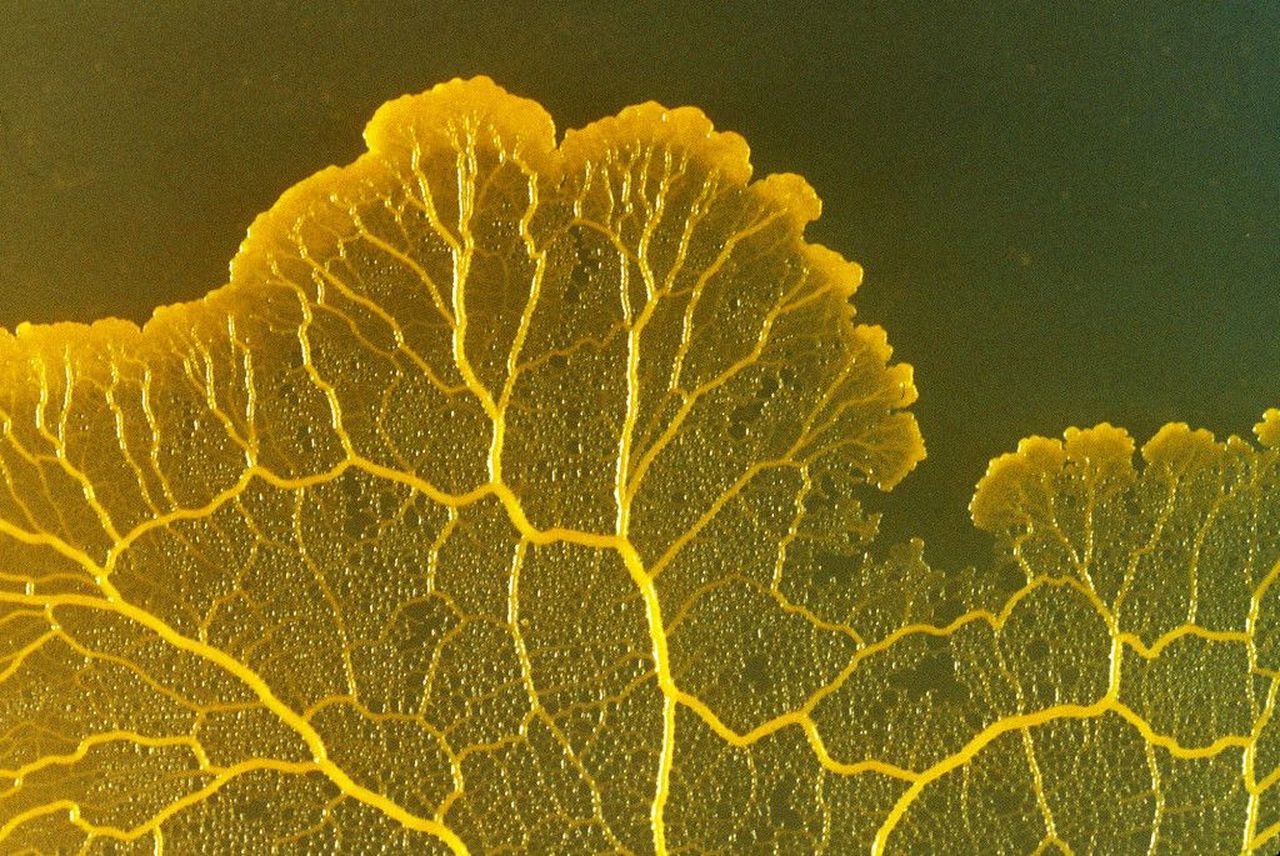
In this investigation, students observe two fascinating attributes of Physarum Polycephalum; the streaming that occurs in the plasmodial form, and the process of phagocytosis. Cytoplasmic streaming is the fan-like leading edges of the organism that form as it moves. This movement can be seen at very edges of the Physarum and through movement of the tubes which lead to these fans. Physarum plasmodium feed through a process of phagocytosis (cell eating), whereby the organism surrounds a food particle and then “pinches'' it into the cytoplasm. Through this feeding process, Physarum plasmodium ingests discrete particles of organic material, bacteria, and other microorganisms. By secreting enzymes, the plasmodium are able to break down substances into their constituent molecules to create smaller particles. The small particles are then consumed by Physarum through pinocytosis (cell drinking), whereby even smaller particles within liquid are consumed. In this investigation, students observe Physarum plasmodium under a microscope and record their observations. They also observe how Physarum responds to the addition of an oat flake in its environment.
PREPARATION - BY LAB TECHNICIAN
Preparing Agar Plates- To melt 2% agar, loosen the agar bottle cap and place it into a beaker on a hot plate filled with boiling water. Prepare enough plates for each workstation. One bottle of agar should be sufficient for five plates.
- After thirty minutes, remove the agar and allow it to cool to approximately 55°C. As the agar is cooling, swirl it occasionally to ensure a smooth even consistency.
- On a sterile workbench, pour the cooled agar into the sterile plates until the bottom of the plate is completely covered. Do not allow the agar to reach over 5mm deep. To reduce contamination risk, open the plates one at a time, only long enough to pour, and then replace the lids immediately.
-
Wait 30 minutes for the plates to solidify before handling or moving them. If an excess of condensation occurs on the lids, you may leave the plates in place with the lids on for 24 hours to dry.
-
Store agar plates in a ziploc bag at room temperature.
Preparing plates of Physarum
-
Approximately one week before the procedure, prepare five plates of Physarum. To do this, cut a 0.5-cm square of Physarum-containing agar from the Physarum agar plate using a sterile scalpel.
- Lift the portion of Physarum-containing agar using your scalpel and position it Physarum side down on a fresh 2% agar plate in the centre.
- Using sterile forceps place approximately twenty-five oat flakes onto each plate. To encourage the Physarum to spread out, place the majority of the flakes in the centre of the plate with roughly six to seven flakes placed towards round the edge. To maintain the sterility of the remaining oat flakes, remove the flake from the packet quickly and immediately close the package.
- Place each plate separately in a ziploc bag and store in the dark at room temperature. Allow five days before using the plates as you need time for growth to occur. However, do not allow the plates to grow for an extended period of time as the Physarum will begin to grow outside of the plate itself.
Preparing Workstations
- Provide each workstation with a small beaker filled with roughly 30% Ethanol or Isopropanol (≥70%). Then place all metal instruments for that workstation inside. The Alcohol only needs to be deep enough to submerge the ends of the metal instruments.
-
Provide each workstation with access to:
-
1 Agar Plate (2%)
- 1 Plate with Physarum Plasmodium (shared)
- Sterile Scalpel
- Sterile Forceps
- Stereomicroscope
- Ziploc Bag
- Oat flakes
METHOD - STUDENT ACTIVITY
Microscopic Observation
-
Collect an agar plate of Physarum plasmodium and place it under the microscope to observe. Begin your observations under the lowest power, gradually increasing it to 30–40×. Keep the lid of the dish closed during your observations to avoid contamination.
- Inspect the Physarum and find the edges of the plasmodium; which will most likely appear fan-shaped. Pay close attention to veins leading to the edges and observe carefully for a few moments. Identify if you can see any movement.
- Record your observations and share the plate with another group.
Subculturing Physarum
-
To make your own plate of the slime mould, cut a 0.5-cm square of Physarum-containing agar from the Physarum agar plate using a sterile scalpel. Ensure sterile technique is followed during this procedure. The work surface and all equipment should be sterilised.
- Lift the portion of Physarum-containing agar using your scalpel and position it Physarum side down in the centre of a fresh 2% agar plate. Replace the lid immediately.
- Place the plate in a sealed ziploc bag and store in the dark at room temperature for 24 hours. If possible, periodically observe the plate over the next 24 hours. Alternatively, inspect the plate after 24 hours. Record what changes you see.
Oat Flake Reaction
-
Collect an oat flake using sterile forceps and position it on the agar 0.5 cm from the edge of the plate.
- Observe your plate at regular intervals over the next 24 hours if possible. If not, observe it 24 hours later. Observe how the slime mould responds to the addition of the oat flake. Determine whether it moves towards or away from the oat flake and Record your observations.
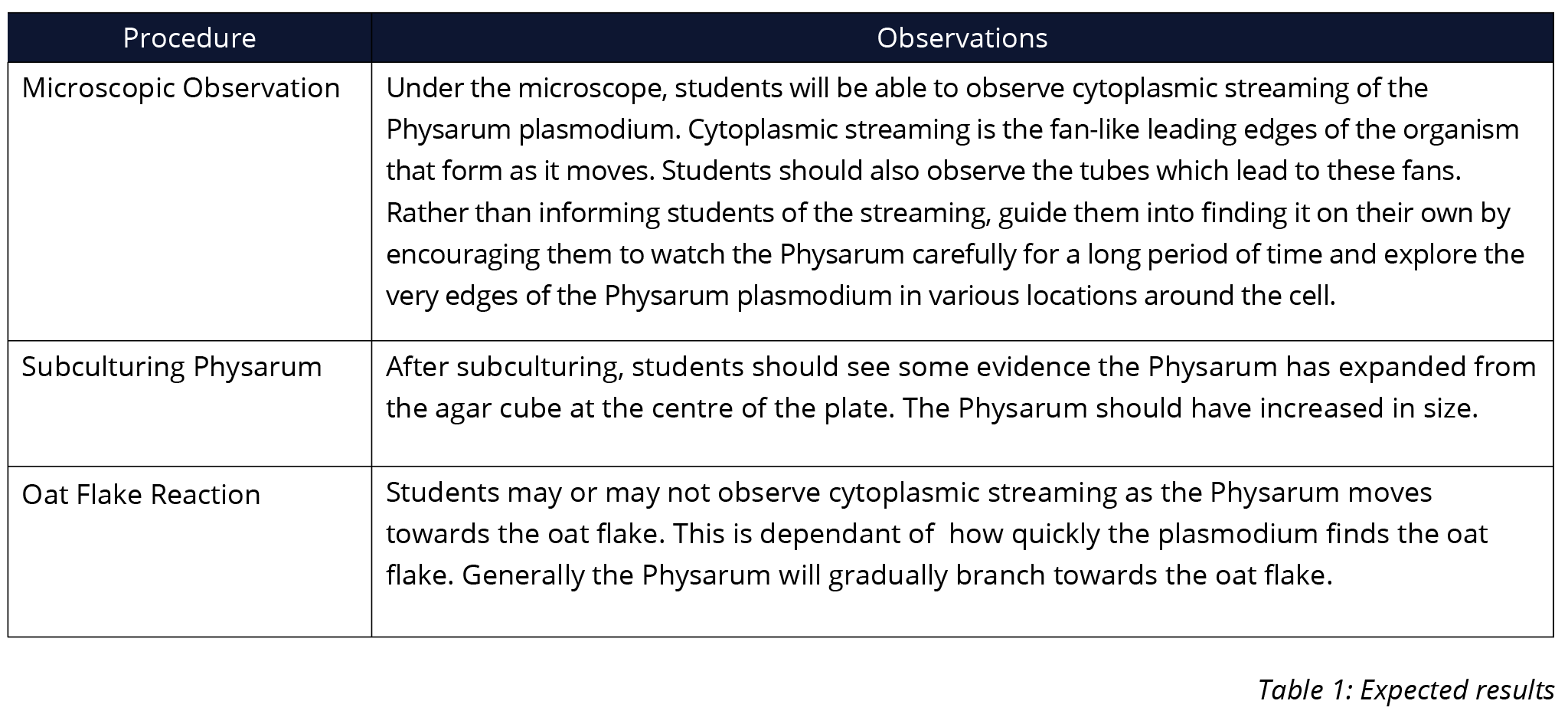
OBSERVATION AND RESULTS
Below is an example of expected results. This is to be used as a guide only as individual results will vary.
INVESTIGATIONS
-
Once students have observed the streaming movement, ask what function they believe it fulfils.
- Ask students to consider if any substances require transportation through the cell. Ultimately, you want students to understand that the streaming and the veins they observed function as intracellular transport and as a mechanism of movement.
EXTENSION EXERCISES
A great extension exercise is to investigate chemotaxis in Physarum. Place two oat flakes equal distance from slime mould in the centre of a petri dish. Instruct students to soak one in a test substance (i.e sugar, salt, cedar oil) and leave the other plain. Students can then observe whether the Physarum moves towards the plain or test oat flake. This behaviour will indicate whether the Physarum is likely repelled or attracted to the test substance.
 Time Requirements
Time Requirements
- 50 mins
 Material List
Material List
- 1 Plate with Physarum Plasmodium (shared)
- 1 Small Beaker (containing 70% Alcohol)
- Sterile Forceps
- Stereomicroscope
- Ziploc Bag
- Oat flakes
 Safety Requirements
Safety Requirements
-
Appropriate Personal Protective Equipment must be worn at all times.
- Wash your hands thoroughly, and clean all surfaces with 70% alcohol before and after the experiment.
- Physarum Polycephalum is not pathogenic; however, it is recommended that all microorganisms are treated as potentially hazardous.
