Living Organism Care- Protists
Summary
- As soon as your shipment arrives, open the shipping container, remove the Protists jars, and inspect your culture .
- Loosen the lids on the jars and aerate the culture using the supplied plastic pipette.
- To bubble air into the water, hold the pipette tip into the culture water and squeeze the bulb. Raise the pipette; releasing the bulb; allowing it to fill with air once again.
- Repeat this step 4 more times to assist in replacing the oxygen depleted during shipping.
- Use a different pipette for each culture to avoid contamination if you have more than one.
- Lightly place the lid back on the jar; careful not to screw too tightly.
- Maintain the culture jar at room temperature (about 21 °C) and away from direct sunlight.
Background
- Protists describes protozoa and other eukaryotes with plant-like characteristics.
- The reproduction of Protists occurs through cell division.
- Protists are microscopic and the majority of them cannot be seen with the naked eye.
- Protists are common on all continents.
- Domain: Eukarya
- Kingdom: Protista (historical)
- Southern biological stocks the following Protists: Paramecium, Amoeba, Euglena and Chilomonas.
Preparation
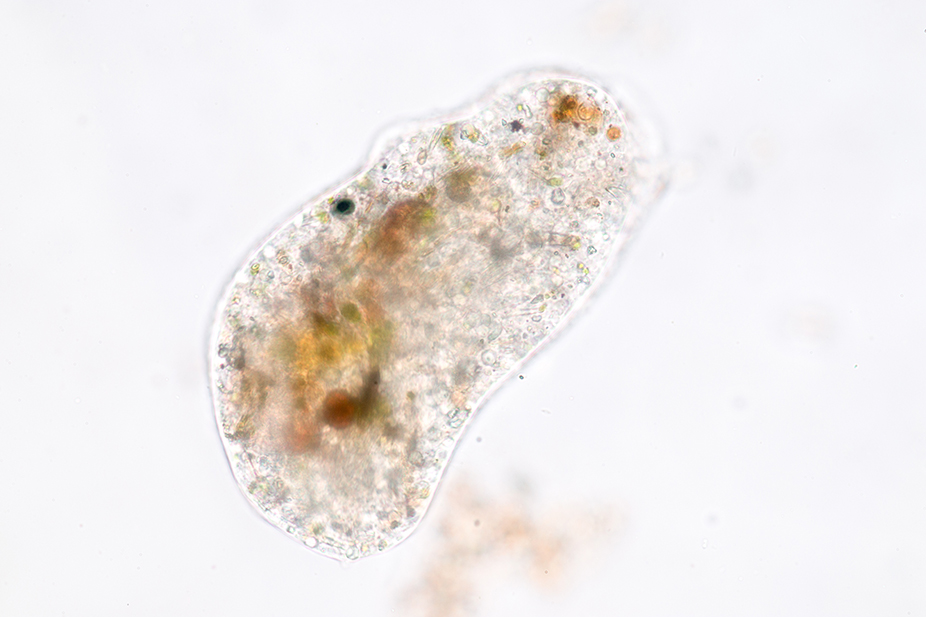
Allow the organisms 15 to 20 minutes to settle after after aeration. Inspect the culture using a stereo microscope and low illumination. The stereo microscope will enable you to identify areas of high organism concentration. These points of concentration are best for students to obtain their samples from when preparing slides for viewing. To pick out single organisms for individual slides, use a stereo microscope and pipette.
A variety of protozoa are easily identifiable as they concentrate in areas of abundant food particles. To identify high concentration areas, you should see a ‘fuzzy debris’ within the culture. Conversely, Amoebas move slowly and lack a constant shape, and thus can be difficult to find. Allow the bottom of the jar to remain undisturbed for 15 minutes and then inspect the bottom of the jar. Using a stereo microscope you should begin several Amoebas as they move slowly over the bottom. Faster moving organisms, such as Chilomonas should also be visible swimming about in the Amoeba culture. These Chilomonas are a food source for the Amoebas.
For standard lab uses, prepare a wet mount to view under a microscope. Squeeze the pipette bulb before placing the pipette tip into the culture. Release pressure from the bulb as the pipette’s tip is near a protozoa concentration area. Maintain the pipette vertical while drawing the protozoa to avoid disrupting the culture and scattering the organisms. For the same reason, do not release and of the pipette water back into the culture. One drop of the sample should have a sufficient amount of organisms for a wet mount. Add a coverslip and use the lowest magnification of the microscope to inspect the slide. Once the protozoa has been located, adjust the magnification to high to observe the organisms in greater detail. Some protozoa, primarily ciliates, move so quickly, keeping them in the field of view is difficult under high-power magnification. There are quieting solutions available that will allow you to slow the ciliates’ movement without damaging them.
Housing
No Housing information needed for this organism
Feeding
The Euglena cultures are Photosynthetic and therefore need a source of light. The Heterotrophic forms (Paramecium and Amoeba) are packaged with food contained within the jar; this food is sufficient enough to maintain the culture for a period of 5-7 days.
Maintaining and Culturing
Photosynthetic protists such as Euglena require need light to manufacture their own food. Ensure these organisms have access to enough light, use either indirect natural light or a light bank. Cultures are not intended to be stored for extended periods, ideally use within 3 days of receiving and do not place within a refrigerator. Cultures should be maintained at room temperature ( Approx. 21 °C), away from direct sunlight, with the lid lightly placed over over the jar unscrewed.
To culture your protists such as Amoeba or Paramecium, pour 20 - 25mL of the Protist culture medium into a very clean glass Petri dish, approximately 100mm in diameter. Add 4-5 grains of uncooked rice. Use a Pasteur pipette to transfer organisms from your culture to the culture medium, replace the lid of the Petri dish, and leave undisturbed in a dark cupboard at 20 - 25oC for a few weeks. The protists will grow steadily in this solution and should be subcultured every four weeks to fresh culture medium.
Note: Other organisms will also be present in your culture, such as chilomonas. Some of these comprise food organisms for your culture and whilst it is important that teachers should point out the correct identity of the amoeba to the students, the intruders are not harming the amoebas and in fact form part of their basic food supply.
Bio Safety:
Southern biological does not supply pathogenic or parasitic protists. Always wash and dry your hands thoroughly after working with any microorganism.
FAQ:
How long will I be able to keep my cultures before using them?
We recommend planning to use the protozoan cultures within a few days of receiving them. Most will last for 5–7 days with minimal care. However, it is important to note, some cultures will survive longer than others. Euglena and Paramecium cultures, have a tendency to live longer whilst, Amoeba survive a shorter period. To determine if the cultures are still usable, inspect them under a stereo microscope.
My cultures arrived Friday, and I need them for class Monday. Will they be okay?
Remove the cultures from their shipping container and care for them as directed above. The worst thing to do is leave them in the unopened shipping container.The Euglena cultures are Photosynthetic and therefore need a source of light. The Heterotrophic forms (Paramecium and Amoeba) are packaged with food contained within the jar; this food is sufficient enough to maintain the culture for an extended period. The cultures health can potentially improve when they are left to rest over the weekend as this will give them to recover from the shipping process.
My students are struggling to find any protozoa. What should I do?
Firstly, check that the students are following the sampling procedures as outlined in the Preparation section. It is important to remember that Protozoan species differ greatly in size; smaller organisms such as Euglena will not be as easy to identify as larger species such as Paramecium. Guide students to look for smaller organisms when attempting to identify Euglena. To locate protozoa concentrations, inspect the culture using a stereo microscope and task students with collecting samples from the areas of concentration.
We used a quieting solution, but the protozoa have migrated to the edge of the coverslip or even slipped out from the coverslip. What should I do?
When solution and culture water are not mixed thoroughly, the thicker solution will displace the water and protozoa with the addition of the coverslip. If this occurs, start over with a clean slide. Ensure you thoroughly mix the quieting solution and culture water before adding the coverslip to the slide.
