Lactose Intolerance

AUSTRALIAN CURRICULUM ALIGNMENT:
- Enzymes have specific functions, which can be affected by factors including temperature, pH, the presence of inhibitors, and the concentrations of reactants and products (ACSBL051)
BACKGROUND:
Enzymes are biological molecules that act as nature’s catalysts. They speed up chemical reactions within cells to make them useful for metabolic pathways. Enzymes are essential to life and have a variety of functions within the body, such as, playing a key role in digestion and metabolism. Unlike chemical catalysts, enzymes recognise substrates (reactant molecules) and convert them into products. While chemical catalysts act on a broad range of reactants, enzymatic reactions involve the enzyme recognising the substrate by its shape and by the position of its hydrogen bonding sites. The enzyme binds the substrate to itself at the active site where the substrate rapidly undergoes the enzyme catalysed reaction. β-galactosidase, also called lactase, beta-gal or β-gal, is an enzyme that breaks lactose down into glucose and galactose by breaking the glycosidic bond.
Lactose is a disaccharide present in the milk of mammal mothers and is commonly consumed in milk and dairy products made from cow's milk. Naturally present within the small intestine, β-galactosidase or lactase enables individuals the ability to successfully digest Lactose. Individuals who do not produce adequate quantities of this enzyme are lactose intolerant and can suffer from discomfort after consumption of dairy products.
In this investigation, students explore the properties and biological function of lactase. They observe the enzyme activity in lactase when exposed to the lactose found in the milk. This enzymatic activity is determined by the formation of glucose. Because glucose is a product of lactose hydrolysis, measuring the amount of glucose gives a direct measurement of how much lactose has reacted. The presence of glucose is determined using glucose testing strips. The colour in the glucose strip changes as a result of the glucose being oxidized to form gluconic acid and hydrogen peroxide. The hydrogen peroxide reacts with a potassium iodide chromogen to change the strip colour from blue through to green and brown in the presence of glucose.
PREPARATION - BY LAB TECHNICIAN
- To make the enzyme solution, add 5mL of lactase solution to 95mL of distilled water. Stir using a stirring rod.
- To make the sucrose solution, add 5g of sugar to 100 mL of distilled water. Stir until the sugar is fully dissolved using a stirring rod.
- To make skim milk solution, add 15 mL distilled water to 1g skim milk powder. Alternatively, you may provide students with 15 mL of fresh skim milk.
METHOD - STUDENT ACTIVITY
- Using a permanent marker, label the four test tubes A, B, C and D.
- A = Milk and enzyme solution (lactase).
- B = Milk and distilled water.
- C = Sucrose solution and enzyme solution.
- D = Sucrose solution and distilled water.
- Using a permanent marker, label the bulb 4 pipettes M, E, W and S. The pipettes must only be used for the substance for which they are labelled.
- M = Milk.
- E = Enzyme solution (lactase).
- W = Distilled water.
- S = Sucrose solution.
- Using the appropriate pipettes, add 2 mL of milk and 1 mL of lactase enzyme solution to Test Tube A.
- After 2 minutes, test for the presence of glucose using a glucose testing strip. You should see a blue square on the testing strip, this contains enzymes that react to the presence of glucose. This reaction can be observed via a colour change.Quickly dip the glucose strip into the liquid, take it out and wait 1 minute. Observe the colour of the blue square. A colour change to green or brown will indicate the presence of glucose. Record the results in Table 1 by simply writing positive or negative.
- Add 2 mL of milk and 1 mL of distilled water to Test Tube B. Following the same procedure, test for the presence of glucose and record the results in Table 1.
- Add 2 mL of sucrose solution and 1 mL of enzyme solution to Test Tube C. Test for the presence of glucose and record the results in Table 1.
- Add 2 mL of sucrose solution and 1 mL of distilled water to Test Tube D. Test for the presence of glucose and record the results in Table 1.
OBSERVATION AND RESULTS
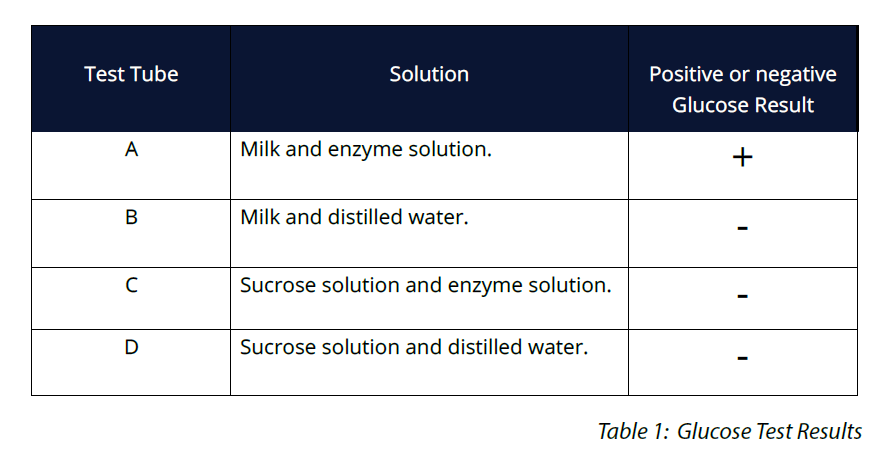
Below is an example of expected results. The test tube containing the milk and enzyme solution is expected to test positive for glucose. The other 3 solutions are not expected to contain glucose.
INVESTIGATIONS
- Ask students to draw a diagram to demonstrate the lactose and lactase reaction. Include annotations of the substrate, the active site, the enzyme, the enzyme-substrate complex, and the products.
- Sucrose, also known as ordinary table sugar, is a disaccharide like lactose. It is composed of glucose and fructose. Sucrose and lactose have the same chemical formula, C12H22O11. Based on this information, ask students why the enzyme reacted to lactose rather than the sucrose.
- Challenge students to identify what type of chemical reaction is lactase speeding up in the above example. Students should identify whether it is hydrolysis or dehydration synthesis.
 Time Requirements
Time Requirements
- 45 mins
 Material List
Material List
- Lactase Solution (5mL)
- Sugar (5g)
- Milk Powder (1g)
- Distilled water
- 4 Test tubes
- 4 Transfer Pipettes
- 4 Glucose Test Strips
- Stirring Rod
- Permanent marker
- Clock/ stopwatch
 Safety Requirements
Safety Requirements
- Wear appropriate personal protective equipment (PPE).
- Know and follow all regulatory guidelines for the disposal of laboratory wastes.
- Wash hands thoroughly before and after handling any chemicals.
- Sterilise work surfaces before and after the practical.
- Under no circumstances are the materials used in this practical to be consumed as food.
- Avoid any direct contact with the solution.
- All solutions can be poured down the sink.
