Enzymes in Action - Lipase
CURRICULUM ALIGNMENT
- Biochemical processes in the cell are controlled by the nature and arrangement of internal membranes, the presence of specific enzymes, and environmental factors
- Enzymes have specific functions, which can be affected by factors including temperature, pH, the presence of inhibitors, and the concentrations of reactants
BACKGROUND
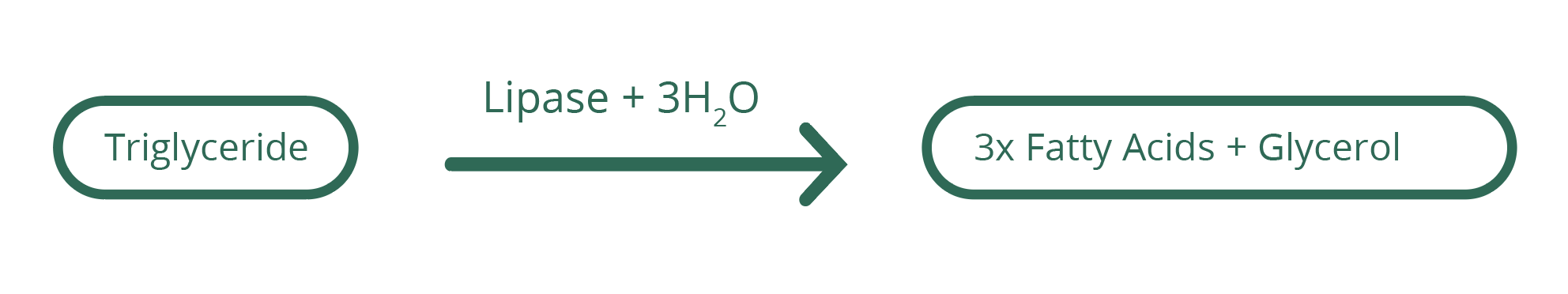
Lipase is an enzyme which works to break down lipids (dietary fats) which are triglycerides; that is, they are esters formed with three fatty acid molecules on a glycerol backbone. Lipase hydrolyses the bonds between the fatty acids and the glycerol, splitting up the triglyceride into fatty acids so that they can be absorbed into the digestive system.
In this practical, students use hydrogen carbonate indicator to observe the drop in the pH of lipid samples as Lipase breaks down the triglycerides into fatty acids. Bile salts are used to emulsify the lipid samples to speed up the rate of reaction. Hydrogen carbonate indicator changes colours in the pH range 7.6 to 9.2, from yellow at the neutral end through orange and red to purple at the alkaline end. Sodium carbonate is used to increase the starting pH into the observable range, however care must be taken with the volume added as a pH of 10 or more can denature the Lipase. It is advised to aim to get the indicator into the red area rather than purple for this reason.
Students can work in pairs or groups, and test a range of lipids or just one type with a range of variables such as temperature. This prac is a great way to explore enzymes with a visual result, and allows the opportunity to touch upon topics such as acidity and alkalinity, hydrophobic vs hydrophilic, surfactants, micelles and surface area.
PREPARATION - BY LAB TECH
- Prepare a 2.5% solution of Lipase by adding 2.5g of Lipase powder to 100mL of distilled water. Stir to dissolve. Each group will need 2mL for each triglyceride to be tested.
- Prepare a solution of 0.01M sodium carbonate (Na2CO3). If you are starting from sodium carbonate powder, first make up 100mL of a 0.1M solution by dissolving 1.06g of Na2CO3 into 100mL of distilled water. Dilute by adding 10mL of the 0.1M solution to 90mL of distilled water to make a 0.01M sodium carbonate solution. Each group will need anywhere from a drop to 4mL for each triglyceride to be tested.
- Prepare a 5% solution of bile salts by adding 5g of bile salts powder to 100mL of distilled water. Stir to dissolve. Each group will need 2mL for each triglyceride to be tested.
- Prepare the hydrogen carbonate indicator if you are starting with the 10x concentrated version by adding 10mL of the concentrate to 90mL of distilled water (adjusting for the volume you need). If you are starting with the already-diluted working solution then there is no preparation required. Each group will need 3mL for each triglyceride to be tested.
METHOD - STUDENT PRACTICAL
- Label your test tubes. For each triglyceride to be tested, you will need two; one for the experiment and one for the control.
- Place 2mL of your samples into your test tubes.
- Add 1mL of 5% bile salts solution to each sample.
- Add 1.5mL of hydrogen carbonate indicator to each sample.
- Give your test tubes a shake to mix the contents as much as possible. If you are using oils, they will still separate once it settles.
- Starting with your control, add 0.01M sodium carbonate. Be careful not to add too much, or the Lipase in your experiment tubes may be inhibited and your experiment will take too long. Add the sodium carbonate drop by drop until the solution changes to a red-purple colour. The colour does not need to be exact, just ensure it has moved away from yellow or there will be no change to see later. If it turns bright purple, you have added too much and might want to start with a fresh sample. Once you have the right amount of sodium carbonate added, add the same amount to your experiment sample. Depending on what you are testing, you may need to add one drop or 2mL of sodium carbonate, or anywhere in between. Milk is slightly acidic, so will probably need 1-2mL of sodium carbonate, and oils are likely to need just a few drops.
- If you are testing more than one substance, do not assume that both will need the same amount of sodium carbonate, even if they are two different types of oil.
- To your control samples, add 2mL of distilled water. Shake to mix as much as possible.
- To your experiment samples, add 2mL of Lipase. Start your timer and shake to mix as much as possible. If you are testing oils, you may need to shake your test tubes periodically to keep the oil mixed in with the other solutions.
- Take note of the time taken until the colour has definitely changed in comparison to the control, and if you have a set of standards you may be able to work out the approximate pH. If you leave the experiment running, it should eventually turn through all of the colours to yellow.
OBSERVATION AND RESULTS
Vegetable oil may only need a few drops of sodium carbonate to edge the pH toward purple, while olive oil may need a drop or two extra. Milk may need one to two millilitres. Vegetable oil may start to change colour almost immediately on the addition of Lipase, assuming that it has not been made too alkaline, and milk may take five to ten minutes. Leaving the experiment for some extra time should deepen the colours and make the changes more obvious.INVESTIGATION:
Discuss triglycerides with your students, in particular that they are made up of three fatty acids attached to a molecule of glycerol. There may be one, two or three different types of fatty acids which make up one triglyceride molecule. Students may be able to recognise that the breakdown by Lipase of the triglycerides into fatty acids is the reason for the drop in pH as the reactions progress.
EXTENSION EXERCISES:
- You may like to compare the rate of activity of Lipase at different temperatures. Place one test tube on ice, another at room temperature, one at about 40oC, and another at room temperature using Lipase which has been boiled for several minutes. We recommend boiling and cooling the boiled Lipase ahead of time. Ensure the enzyme, your sample, the sodium carbonate and the indicator are all at the same temperature by putting them all in the relevant water or ice bath for a few minutes before combining them. This is particularly important for the ice station, because if you add cold Lipase to warmer milk, the enzyme might heat up enough and work faster than expected. The exception is the boiled-enzyme station, as nothing else needs to be boiled - room temperature will do. Ask your students what they would expect to be the ideal temperature for a mammalian enzyme. They should understand that mammalian bodies are generally kept in the range of 37-39oC and so the enzymes used would also work best in those temperatures. Commercial enzymes, however, are often harvested from other sources such as fungi or bacteria and stability and so the ideal temperature may vary. In general, cold temperatures will slow enzymes down, the activity increasing with increasing temperature until a high enough temperature is reached that it is denatured and will no longer work at all.
- Another great extension exercise is to test how an absence of bile salts affects the experiment. To do this, place Lipase in both test tubes, but replace the bile salts with the same volume of water in the control test tube. Bile salts reduce the particle size of the fat droplets which increases the surface area, thereby allowing better access for the Lipase to break down the triglycerides faster. Discuss the use of bile in the digestive system to solubilise lipids due to its amphiphilic nature - that is, that it has a hydrophilic head and a hydrophobic tail. Bile will organise itself around droplets of fat with the tail pointing inwards and the head outwards into the water, preventing the droplets from merging back with other droplets of fat. This is called a micelle. These smaller droplets have a larger surface area, which means there is more of the lipids exposed to the enzyme, increasing the rate of reaction.
 Time Requirements
Time Requirements
- 40 min
 Material List
Material List
- Lipase solution 2.5% (w/v)
- Sample of Triglycerides (olive oil or full cream milk)
- Bile salts solution 5%
- Hydrogen Carbonate Indicator
- Sodium Carbonate solution, 0.01M
- Distilled Water
- Transfer Pipettes
- Test Tubes
 Safety Requirements
Safety Requirements
- Wear appropriate personal protective equipment (PPE).
- Know and follow all regulatory guidelines for the disposal of laboratory wastes.
- Be aware of any food allergies or sensitivities to chemicals.
- Under no circumstances are the materials in this prac to be consumed as food.
- Do not breathe in powdered enzymes. If you spill enzyme solution, clean up with water and paper towels. Do not allow it to dry out as it can cause a fine dust which can be hazardous if breathed in.
 Downloads
Downloads
