Enzymes in Action - Catalase
AUSTRALIAN CURRICULUM ALIGNMENT
- Biochemical processes in the cell are controlled by the nature and arrangement of internal membranes, the presence of specific enzymes, and environmental factors
- Enzymes have specific functions, which can be affected by factors including temperature, pH, the presence of inhibitors, and the concentrations of reactants
BACKGROUND
Normal oxidative cell processes in living organisms, including bacteria, plants and animals, can lead to the formation of hydrogen peroxide, H2O2. This can be very damaging as it is soluble in water and attacks proteins, cell membranes and DNA. To combat the ever-present threat of hydrogen peroxide, living cells produce an enzyme known as Catalase that rapidly breaks hydrogen peroxide down into water and oxygen. This experiment, studies the effect of substrate concentration on the rate of reaction.

Discs of porous filter paper are soaked in a solution of catalase then submerged in a beaker containing hydrogen peroxide. As the reaction proceeds, tiny bubbles of oxygen form and coalesce into larger bubbles. Eventually, there will be enough bubbles to make the paper disc buoyant and it will lift off the bottom of the beaker and float to the top. The time from submersion to “lift off” is a measure of the rate of reaction.
PREPARATION - COMPLETED BY LAB TECHNICIAN
Catalase Solution:
- Prepare a 5% stock solution by mixing 1mL of catalase with 15mL of distilled water, then make up to a total of 20 mL with distilled water.
- Prepare your 0.05% working solution by mixing 1mL of 5% stock solution with 90mL distilled water, then make up to a total of 100mL with distilled water.
Hydrogen Peroxide test solutions:
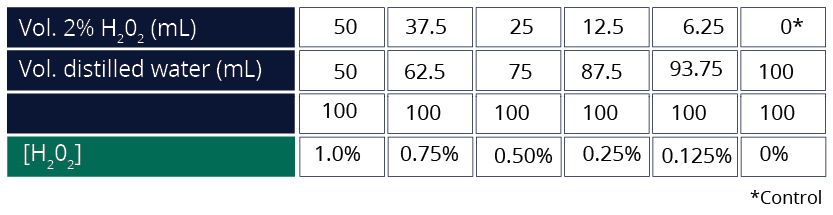
- Prepare a 2% H202 stock solution as follows:
Vol distilled water = 67 mL
100 mL
Prepare test solutions as follows:
Filter Paper Discs
- Make small discs from your filter paper using a clean hole punch.
METHOD - STUDENT PRACTICAL
- Place 16 paper discs in the beaker of Catalase solution.
- Use the tweezers to remove one paper disc from the Catalase. Brush both sides of the disc against the rim of the beaker to remove excess Catalase.
- Swiftly lower the disc into the control beaker and place it on the bottom. It should remain there throughout the experiment as there will be no gas producing reaction. At the conclusion of the experiment, you can note the reaction time as infinity.
- Take a second paper disc from the Catalase solution, brush off excess and place it at the bottom of the 0.125% hydrogen peroxide beaker.
- Start the timer as the disc goes below the surface. Watch the disc closely as it becomes coated in small bubbles and becomes buoyant. Stop the timer at the moment the disc “lifts off” the bottom of the beaker. Repeat step 4 two more times for a total of 3 data points and note the average time for “lift off”.
- Move on to the remaining four hydrogen peroxide beakers, using three discs per beaker and averaging the time for “lift off” in each case.
- Plot [H2O2] on the vertical axis and the reaction rate (1/t) on the horizontal axis.
EXTENSION WORK
-
To study the effect of enzyme concentration on reaction rate; soak 3 paper discs in each of a range of beakers containing
Catalase at different concentrations (0.0125%, 0.025%, 0.05%, 0.075% and 0.10%) and testing each on a 0.25% solution of hydrogen peroxide. - Plot [Catalase] on the vertical axis and the reaction rate (1/t) on the horizontal axis.
- Study the effect of temperature on the reaction rate by preparing 2 beakers; one containing 30mL of 0.25% H2O2 and the other containing 10mL of 0.0125% Catalase and 3 paper discs. Condition the beakers at room temperature and measure the time for “lift off”.
- Repeat this experiment by conditioning the beakers in water baths at various temperatures such as 0°C (slurry of ice in water), 35°C, 40°C, 45°C, 50°C. Reliable results can be difficult at higher temperatures as the hydrogen peroxide breaks down at higher temperatures. Watch for the spontaneous formation of bubbles in the hydrogen peroxide beaker.
- Study the effect of temperature on the stability of Catalase. Enzymes are denatured by exposure to heat. In this study, you set up a beaker of 0.25% hydrogen peroxide at room temperature and a beaker of 0.0125% Catalase containing paper discs on a hot plate with a thermometer inserted. Measure the time for “lift off” when the Catalase is at room temperature, then turn on the hot plate and take more measurements every 10°C. You can expect to see the time for “lift off” increase to infinity once the temperature of the Catalase has reached the temperature at which it denatures.
FUN FACT
- Whereas digestive enzymes are located in specific points of the body, catalase is found in almost all living cells. Discuss the reasons for this in terms of how hydrogen peroxide can be formed in cells and why it must not be allowed to accumulate.
 Time Requirements
Time Requirements
- 40 min
 Material List
Material List
- Catalase solution 5% (w/v)
- Filter paper
- Fine Tipped tweezers
- Stop watch
- Hydrogen peroxide
- Beaker 200ml
 Safety Requirements
Safety Requirements
- Apron required
- Safety Gloves Required
- Safety Goggles Required
