Chemicals of Life

AUSTRALIAN CURRICULUM ALIGNMENT:
- In eukaryotic cells, specialised organelles facilitate biochemical processes of photosynthesis, cellular respiration, the synthesis of complex molecules (including carbohydrates, proteins, lipids and other biomacromolecules), and the removal of cellular products and wastes (ACSBL049)
- Cells require inputs of suitable forms of energy, including light energy or chemical energy in complex molecules, and matter, including gases, simple nutrients, ions, and removal of wastes, to survive (ACSBL044)
- Conduct investigations, including microscopy techniques, real or virtual dissections and chemical analysis, safely, competently and methodically for the collection of valid and reliable data (ACSBL032)
BACKGROUND:
The chemicals in cells can be grouped into four basic categories: carbohydrates, proteins, lipids and nucleic acids. Living cells also contain other chemicals, such as: water, salts and minerals. The four major classes of biological molecules can be identified through simple characteristic tests. By mixing 2-4 drops of iodine solution to 12 drops of ‘unknown solution’, we can identify polysaccharides such as starch. The solution will turn from a yellow-brown to dark purple when polysaccharides are present. Similarly, adding 5-10 drops of Biuret Test Solution to 12 drops of ‘unknown solution’ will illustrate the presence of proteins as the solution turns from light blue to purple. 12 drops of an unknown solution are added to 5 drops of Sudan III and mixed vigorously to determine if lipids are present. In the case of lipids are present, two layers will form with the top layer turning a pale pink-orange colour. The solution will turn pale yellow if no lipids are present. To determine if nucleic acids are present, the process requires 6 drops of an unknown solution and 12 drops of diphenylamine to be mixed and then placed in a water bath for 10-20 minutes. If DNA is present, the solution will turn purple; turning green if RNA is present.
In this practical, students will mix the various solutions to the unknowns to reveal the presence of examples from the four basic categories of cell chemicals. This is an excellent opportunity for students to practice chemical testing, analysis and learn more about the chemicals that sustain life.
PREPARATION - BY LAB TECHNICIAN
Preparing the 'Unknown' Solutions- Increase or decrease quantities in proportion to class size.
- Unknown A: Distilled water
- Unknown B: Mix 1g of gelatin into 100 mL of hot distilled water.
- Unknown C: Mix 1g of soluble starch into 100 mL of hot distilled water.
- Unknown D: Mix vegetable or mineral oil with an equal volume of water.
- Unknown E: Mix 1g of DNA into 200 mL of distilled water.
METHOD - STUDENT ACTIVITY
Testing for Carbohydrates, Proteins, Lipids and Nucleic Acids
- Label the 5 microcentrifuge tubes with the letters A–E.
- Add 12 drops of the respective unknown solution to each of the 5 tubes.
- Add 2–4 drops of Iodine to each test tube to test for the presence of carbohydrates.
- Draw a table to record the results of each test conducted on the five ‘unknowns’, and record test results on the table using (+) to indicate a positive test and (–) to indicate a negative test.
- Remove the unknowns and carefully clean the test tubes.
- Refill the tubes with the five unknowns and repeat until you have tested for carbohydrates, proteins, lipids, and nucleic acids.
OBSERVATION AND RESULTS
At the conclusion of the practical, students should be able to identify which tubes contained carbohydrates, proteins, lipids and nucleic acids through changes in colour and separation of the liquid into the layers. The results should match those in the table below.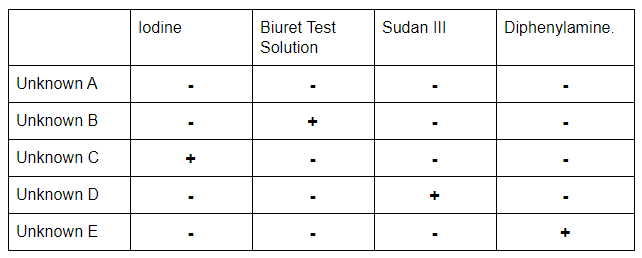
- Unknown A is negative for all tests
- Unknown B is positive for proteins
- Unknown C is positive for carbohydrates
- Unknown D is positive for lipids
- Unknown E is positive for nucleic acids.
If a positive DNA test result is not readily seen, advise students to place the microcentrifuge tube containing the DNA and Diphenylamine solution in a beaker of gently boiling water for 10–20 minutes. A positive (blue or purple colour) should be seen during this time. Be sure the tube stays securely fastened. Alternatively, place the DNA and diphenylamine test solution in an open test tube in the boiling water bath.
TEACHER TIPS:
-
You may like to prepare students for this practical by asking them to run ‘known’ solution tests. This way students can familiarise themselves with what the results of the different tests will look like; making it easier to identify results during the ‘unknowns’ activity.
- Tubes containing vegetable oil are difficult to clean. To save time in class, use fresh tubes to replace them.
 Time Requirements
Time Requirements
- 45 mins
 Material List
Material List
- Distilled Water
- Gelatin
- Soluble Starch
- Mix Vegetable or Mineral Oil
- DNA
- Beaker, 250-mL
- Glass Measuring Cylinder
- Biuret test solution, 25–50 drops
- 5 Microcentrifuge tubes
- Diphenylamine DNA Test Solution, 60 drops
- Pipette
- Iodine solution, 10–20 drops
- Stirring Rod
- Sudan III solution, 25 drops
- Weighing dish or paper
- Portable Balance, 220g Capacity
 Safety Requirements
Safety Requirements
- Wear appropriate personal protective equipment (PPE)
- Exercise caution when handling the chemicals used in this prac as they may cause moderate skin and eye irritation, or even severe burns.
- Avoid any direct contact with the solution and wash hands thoroughly.
- Keep Diphenylamine DNA test solution and Sudan III solution away from open flames as they are flammable.
- Do not inhale any mist, vapours or spray.
