Cauliflower Cloning

BACKGROUND
First devised by scientists in the 1950s, plant tissue culturing methods are used widely in commercial horticulture, as well as studies of biochemistry, cell biology and genetics. Plant tissue culturing involves growing individual plant cells or tissues in aseptic conditions. While plant cells may be sourced from any part of the plant; young, actively-dividing (Meristematic) cells give the best results. When a small piece of a plant (explant) is placed on a sterile growth medium containing the right nutrients such as sucrose, these cells are triggered into growing and dividing. With access to sufficient light, the explants produce leaves and roots as they develop into small plants. Micropropagation techniques using plant cultures enable the production of large quantities of disease-free, genetically-identical plants (clones). Cloning is used increasingly in plant conservation. Scientists are working to re-populate rare and endangered species of plants by growing larger quantities of plants from relatively small amounts of starting material to enable re-introduction into their original habitats. For instance, a small tree species native to Mauritius, Cylindrocline Lorencei, which became extinct in the wild in 1990, was brought back to life through the use of cloning. Scientists cultured plants from the embryos in seeds, and 15 rooted plants were eventually sent back to Mauritius for re-introduction.
In this investigation, students use plant tissue from a cauliflower to grow a genetically identical plant (clone). Cauliflower is the ideal plant for students to work with as it is accessible, fast growing, robust enough to withstand handling and the meristematic tissue from (the tips of the white curd) is easy to isolate. The techniques used in this investigation are adapted from those developed for plant scientists in the field. Students are tasked with cutting small cauliflower florets into smaller explants and sterilising them using Sodium Dichloroisocyanurate (SDICN). The explants are then planted in agar plant growth media where students will observe their growth over a period of three weeks. After three weeks the cauliflower callus tissue develops small shoots, providing students with their own cauliflower clone. In this investigation, students gain practical experience in plant tissue culturing and gain a deeper understanding of plant cells and genetics.
PREPARATION - BY LAB TECHNICIAN
Preparing Kinetin Solution
-
To make Kinetin solution, dissolve 10mg of Kinetin powder into 10mL of 70% Ethanol.
Preparing Milton (SDICN) Solution
-
To create a SDICN solution, dissolve 4 Milton tablets in 400mL of distilled water. Note: Our required SDICN solution is stronger than
what is recommended on the packet, so please ensure you use the above desired concentration. - Fill one vial per workstation with approximately 20mL of the SDICN solution. This vial will be used by students to sterilise their explants. Label these vials ‘SDICN’.
- Set aside 40mL of SDICN solution to be used for the plant growth medium.
- Using the remaining SDICN solution, add approximately 25 mL to one open container per workstation for students to sterilise their forceps. Label this container ‘SDICN’.
Preparing Plant Growth Medium
-
Pour 960mL of distilled water into a large beaker. The vessel needs to be large enough so that the aliquoted liquid does not fill beyond the halfway point. This method makes 1L of plant growth medium, which is enough for 100 vials.
Note: The excess medium may be stored in a large sterile container within the refrigerator for up to 3 months. - Add 4.4g Murashige and Skoog powder and continuously stir until it has been fully dissolved.
- Add 20g of granulated sugar and stir until it has dissolved.
- Using a pipette, add 2.5mL of Kinetin solution into the medium and stir until dissolved.
- Measure the pH either using pH paper or a pH meter and adjust the pH to pH 5.7 using 0.1M Potassium hydroxide
- Add 7g of plain agar powder to the beaker and stir well.
- Cover the beaker in cling film and poke holes into it. This will allow steam to escape when the solution is heated in the microwave. Consider whether the beaker will fit within your microwave oven; if not, you can make the medium in 500mL batches.
- Heat the beaker in the microwave on high power for 90 seconds, stir, re-cover and heat for another two minutes until the agar is full dissolved and the solution has boiled. Do not leave the solution unattended as the liquid may boil over.
- Allow the medium to cool to until it reaches 50 degrees. This should take approximately 30 minutes. Use a thermometer to check the temperature of the solution.
- Working in a well-ventilated area or a fume cupboard, add 40mL of SDICN solution (the sterilant) to the beaker. Stir well until combined. For your safety, avoid breathing in any chlorine fumes and wear gloves when handling the sterilant. Once the sterilant has been added, the medium should be dispensed immediately.
Dispensing the medium
- The medium must be dispensed into sterile vials in sterile conditions. Wipe down your workbench with 70% Ethanol.
- Remove lids from the sterile vials and place the lids facing downwards on a sterile ceramic tile to avoid contaminants dropping into it.
- Pour approximately 10mL of the medium into each sterile vial and allow them to cool for approximately an hour with lids ajar.
- Once the medium has set, replace the lids and label the vials ‘Growth medium’. Note: The vials and any excess medium may be stored within the refrigerator for up to 3 months.
Preparing Cauliflower
- Purchase a fresh, whole cauliflower. Do not use ready-prepared cauliflower pieces.
- Using a sterilised scalpel, cut the cauliflower into small florets; approximately 1cm3. Cut the cauliflower as close to class time as possible to ensure fresh samples.
METHOD - STUDENT ACTIVITY
Preparing for Cloning
-
Put on appropriate PPE; including disposable gloves. Sterilise your forceps by placing them in the open container of solution labelled SDICN.
- Using a paper towel, wipe down your bench with 70% Ethanol.
- Collect your cauliflower floret, place it in a petri dish or on a sterilised tile and carefully cut length-ways using a scalpel to create a small 3-5mm³ piece for explanting.
Sterilising the explants
-
To sterilise your explant, place it in the small vial of SDICN, using your pre-sterilised forceps.
- Replace the lid on the vial, and swirl the container gently for five seconds.
- Continue to gently swirl the container for five seconds every two to three minutes for a total of 15 minutes.
Transferring explants to medium
-
Strain the liquid from the container into a waste beaker. Use your forceps to stop the explant from falling into the beaker. Place the forceps back in the open container of SDICN.
- Remove the lid from your vial of growth medium. Place the lid face down on a clean ceramic tile. Collect the explant using your forceps and transfer it to the growth medium vial. Press the stalk slightly into the medium to secure it in place. To minimise contamination risk, avoid leaning over your working area.
- Replace the lid and label the vial with your name and the date using a permanent marker.
Incubating and observing
-
Incubate the explant in the medium in a well-lit area. The explant should be placed near a window or under a light bank for
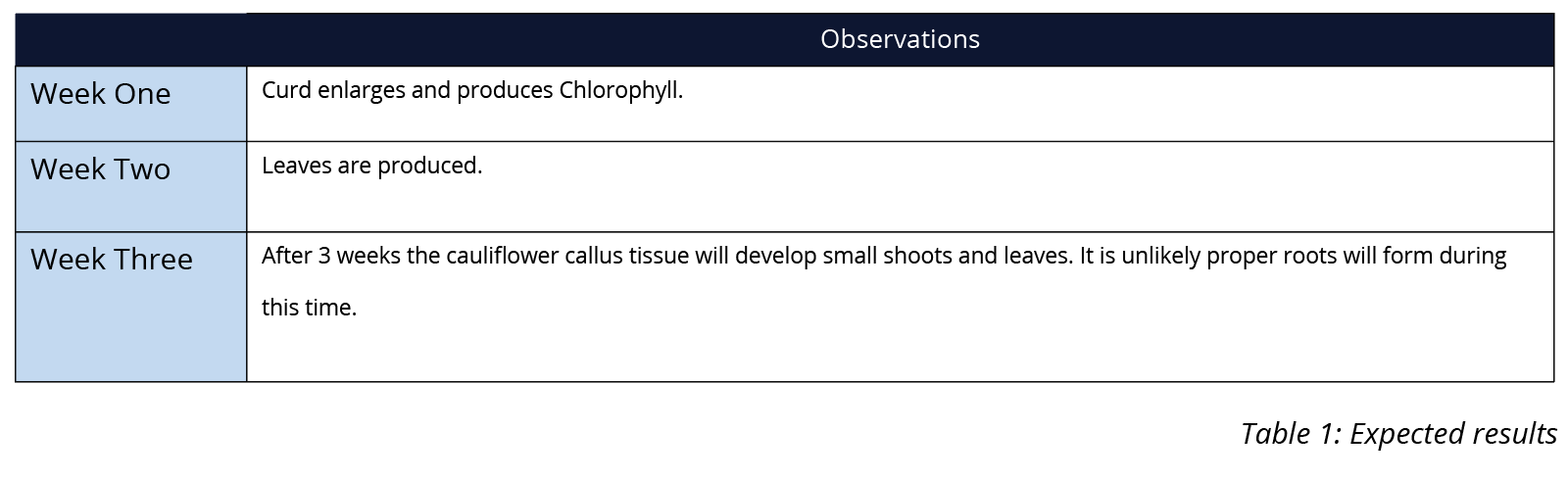
best results. - Examine the culture weekly (or more frequently where possible) over a period of three weeks. You may also like to keep a photographic record of the cultures for future reference and comparison. You should expect to see the beginnings of greening of the explant and growth within ten days. Record your observations in a table like Table 1.
OBSERVATION AND RESULTS
The below table is an example of expected results. It is to be used as a guide only as individual results will vary.
INVESTIGATIONS
- Ask students what steps in the procedure prevent contamination and how.
- Challenge students to describe the role/s sugar, Kinetin, Murashige and Skoog, agar and SDICN (Milton) play in the plant growth medium.
- Discuss what a clone is. Discuss what contexts within biology the word 'clone' used.
- Ask students why is it necessary to place the explants in the vials in well-lit areas.
- Challenge students to describe the advantages and disadvantages of using this method in the cultivation of new plants.
EXTENSION EXERCISE
You may like to give students the opportunity to observe the cloned cauliflower reach full growth. After three weeks the cauliflower will develop small shoots and leaves. To observe further growth, the culture must be placed in a new vial of MS (Kinetin free) growth medium to encourage the formation of roots. After six to twelve weeks the roots will grow, and the small cauliflower plantlets can be transplanted into a fine seed compost to grow into a full-sized cauliflower. To grow the cauliflower successfully, wash the agar off the roots and plant them in a humid environment by attaching a clear plastic bag over the plant pot. The leaves of tissue-cultured Cauliflowers that have been grown to full term in humid conditions lack a fully-developed waxy cuticle and their stomata also responds too slowly to prevent desiccation. For this reason, the plants must be moved after ten days to acclimate to a normal environment.
 Time Requirements
Time Requirements
- 50 mins
 Material List
Material List
- 1 Small Cauliflower floret (10mm3)
- 1x Plastic forceps
- 1x Petri Dish
- 70% Ethanol (Sterilising Bench)
- 1 open container half-filled with with SDICN for sterilising forceps (shared)
- 1x 30mL vial for SDICN (for sterilising explant)
- 1x 30mL vial for growth medium
- Scalpel
- Paper Towels
- 1 White Ceramic Tile
- 1 Permanent Marker
- 100mL Beaker (Waste)
SDICN (400 mL):
- 4x Milton® Tablet (SDICN)
- 400mL distilled water
Growth Medium (1L):
- 4.4g Murashige and Skoog (MS) powder
- 7g plain agar powder
- 20g Granulated Sugar
- 10mg Kinetin powder
- 10mL Ethanol (70%)
- 40mL SDICN solution
- Large glass beaker (making media)
- Small glass beaker (pouring media)
- 1x Pipette
- Stirring rod
- Thermometer (media temperature)
- pH meter or pH paper
- 0.1M Hydrochloric acid & 0.1M potassium hydroxide (for adjusting pH)
- Safety glasses
- Disposable gloves
- Chemical balance (measuring powders)
- Cling-film
 Safety Requirements
Safety Requirements
- Wear appropriate Personal Protective Equipment (PPE), including disposable gloves and lab coat.
- Take care when handling scalpels.
- SDICN is generally safe to handle; however, in this investigation it is being used at several times the recommended concentration. Do not allow the solution to come into contact with eyes or skin. Do not inhale the Chlorine vapours.
- Plant tissue cultures may become contaminated with microorganisms. If this does occur, the vial must not be opened and should immediately be placed in an autoclave bag.
